Many rye breads include a rye sour (a single or multi-stage preferment of rye flour inoculated with a sourdough starter). Along with that, you will regularly read that rye breads benefit from the sour and the acidity it brings to the dough. However, "acidity" is a loosely used term.
For rye breads and when someone states the bread benefits from acidity, is the benefit from the flavors imparted by LABs when they produce lactic and acetic acid, or is the benefit more in the dough properties as a function of pH? You will often read that the flavor profile of a rye bread changes in the first 24-48 hours. What's driving that change? The presence of LAB's during fermentation, the pH of the dough during fermentation, or ????
What's driving my question is when I want to make a 100% rye bread...
- I have a rye starter. It is rarely used and horribly neglected.
- I have a white flour starter that is refreshed regularly. I know I can take it and do a few refreshes with 100% rye and functionally make a rye starter.
- I also have a blueberry yeast water that is regularly refreshed and active.
- If the benefits are driven by LAB's, that would suggest a sourdough starter is required. However, if the benefits are pH driven, my yeast water naturally has a pH below 4.0. I haven't tested it yet, but I'm guessing a rye sour inoculated with yeast water and allowed to ferment for 12-24 hours would get a pH in the 4.0 - 4.5 range. The initial pH would be low from the yeast water and then the subsequent drop would come from the activity of microorganisms present in the rye flour. I think the final pH values would be similar to a rye sour inoculated with starter but the yeast/LAB profile would likely be quite different.
Long story short... Can I let my rye starter go out to pasture, skip all the refreshes it takes to convert a white starter to a rye starter, and just use my yeast water as 100% of the hydration for a rye sour?
According to "Bread" and "The Rye Baker" the acids in the sourdough culture moderate the functioning of the amylase enzyme that breaks down starch into sugars. Without the acid, the high amount of amylase in rye would break down starch too fast, making the crumb gummy - the dreaded "starch attack".
So if you have a lot of rye in the dough, you want to ferment the sourdough to favor the production of the LAB.
I think any nicely active starter would work, but make sure the first stage is at a temp and time that favors LAB
That said, my 100% dark rye culture seems to revive after a week in the fridge with one refresh and 12 hrs at room temp. If it has been neglected longer, 2-3 refreshes seem to bring it back pretty well.
Thanks! This sounds like it's pH driven and not necessarily lactic/acetic acid driven. Anything that suppresses pH should suppress amylase.
Rye bread dough is no different from wheat, Troy. It can be made with preferments or by relying on a straight dough method. The choice is yours depending on your schedule.
I usually prepare rye preferments overnight, 8-12 hrs at 90F, mix rye bread dough at 6AM and have a fresh loaf of 100% rye bread for breakfast, two hours later, warm from the oven. My rye starter is like your yeast water, it is flourless, but it has both yeast and lactic acid bacteria in it and the resulting rye preferment is very sour, very, when ready, while bread dough and bread are only mildly sour, very pleasant to taste.
The general function of preferments in rye dough is the same as in wheat dough: modify flour, accumulate flavor and acid load, activate yeast (and sd bacteria), manage time.
https://bakerpedia.com/processes/preferment/
Strictly speaking, rye bread does not require sourdough or souring and can be made with yeast or soda alone, but it depends on the quality of rye flour. Many kinds of rye flour have such low falling number that they will bake into a gummy brick, or even into a hollow bread with a layer of fudge at the bottom crust, they need acidity. North American rye flours are mostly like #6 in the pictures below, with high falling number, 200seconds and higher, very low in sugar content.
Also, rye flour is so rich in harmful bacteria and mold spores, it needs sourdough's antibiotic properties to improve rye bread's keeping qualities, to suppress those spores. It is needed not so much for sour taste but for normal crumb.
Soft and sweet rye flours that give gummy crumb are exceedingly rare in the US and Canada but common in Europe, that is why they need rye sours (rye preferments) or rye starters if made by direct method..
As for replacements, whether you can use apple juice or Pepsi or yeast water or yogurt or sour beer, or any other sour liquid in your recipe instead of plain water, this you should try and see. If your flour has a high falling number, it won't make any difference in quality of bread crumb, only in aroma and taste and in keeping quality of bread..
pH has zero importance in rye baking, that I know for sure, although some rye breads are traditionally very sour to taste (low pH and high acetic acid content) and others are traditionally very mild (high lactic acid content regardless of pH). TTA is very important. My biggest problem in the past was exactly that, I would reach a decent pH in my rye sours and rye dough but not high enough total titratable acidity and the bread was just meh, really not interesting nor good looking. I struggled with that issue (of ugly 100% ryes) for years until I figured it out.
You don't want that activity, believe me. The whole point of using sourdough in rye (or pineapple juice or whatever acidifier is used in initial 24-48hrs of sd starter development) is to suppress it! Or else your bread will stink to heavens.
Your interesting post (it sent me to the web to learn about falling number) includes this "pH has zero importance in rye baking". This seems to contradict some of the other things in your post about the use of acidity (which is measured by pH; pH = 7 is water, lower numbers more acidic, higher numbers more alkaline/basic) to slow down the amylase which breaks down starch into sugars. If you need acid to slow the amylase, that's the same as saying that you need a lower pH.
You can use rye flour in bread with commercial yeast; sourdough culture or other acids let you use a higher percentage of rye flour without the gumminess.
in "The Rye Baker" and "Bread", many of the sourdough rye recipes are spiked with commercial yeast for lift; the sourdough culture is mainly for its acidity.
There is a subtle difference between pH and TTA, and it is made complicated by the type of acids that are present in bread doughs: mainly lactic an acetic acids.
Lactic and acetic acids are considered weak acids, in that they do not fully dissociate into protons (H+) and lactate or acetate ions when dissolved in water. The impact of this is that these acids will produce higher pH values than an equivalent amount of a strong acid (e., hydochloric acid, HCl) when dissolved in water.
For example, I added 1 mL of distilled white vinegar (5% acetic acid) to 20 mL of DI water; I measured pH 3 with pH paper, which is close to what I have seen reported for this concentration. An equivalent concentration of HCl in water will yield pH 1.3.
What does this mean for bread dough? Higher amounts of these acids can accumulate in the dough (i.e., TTA) without affecting pH as much as HCl would. These weak acids naturally create pH buffers that moderate the pH that is achieved during dough development. Higher amounts of these acids will produce more flavor, as @mariana stated.
Here some videos from Puratos that illustrates these concept:
https://youtu.be/de0qJIoQMRg
https://youtu.be/sjlxxStd_Nw
https://youtu.be/ZZmwt6Ug-Zg
https://youtu.be/l_8UDwFETZo
Here is another interesting observation that I made. I added 4 mL of distilled white vinegar to 80 mL of my tap water; this solution had pH 3.5. Why? Because my tap water has some dissolved carbonates and bicarbonates, etc., that neutralize some of the vinegar. That could have some bearing on pH and TTA of my sourdoughs.
My local tap water has a high pH level due to it being hard water. Could very well explain the difficulties when it comes to rye and sour. Now you have put it like that i've had a lightbulb moment. Something to explore and experiment with.
You can purchase Burton salts in homebrew stores here to mimic the hard, alkaline water used in breweries in Burton-on-Trent. Sounds a little like your water.
I don't actually know what the pH is of my water as my pH papers only go up to 6.
Just looked up the details of my local water. Does this mean anything to you? I know it's high but how does this translate into pH?
I’m not sure you can make a direct correlation to pH from hardness. Do they also report alkalinity?
And haven't found any conversion rate between hardness of the water and pH level. I'll have to have another look at the report.
This article says hard water is pH > 8.5
https://www.atsenvironmental.com/residential/water/contaminants/list/ph-levels/#:~:text=Water%20is%20considered%20hard%20when,such%20as%20tea%20or%20coffee.
Here is another on hardness and pH
https://opentextbc.ca/ingredients/chapter/water-hardness-and-ph/
It also says:
Effects on Baking
Most municipal supplies of water contain chlorine, which is used to ensure the purity of the water. Some cities add fluoride to their water supply to stop tooth decay. Neither chlorine nor fluoride is present in large enough quantities to affect dough in any way. In addition, most municipal water is treated to reduce excessive acidity, since this could be corrosive for the water lines. It is therefore unlikely that bakers using municipal water need to be concerned about extremely acidic water.
Soft water is another matter, as it can lead to sticky dough. An addition of yeast food, or a reduction in dough water, will help. Alkaline water tends to tighten the dough and retard fermentation, since enzymes work best in slightly acidic dough.
Hard water does not necessarily have to be basic. Hardness is due to the presence of calcium and magnesium cations, but they can be paired with other anions (sulfate, nitrate, chloride) that will not contribute to alkalinity or higher pH.
However, most hard water in the Midwest is due to the presence of limestone or dolomite in the bedrock, and these will contribute to alkalinity. I haven't measured my pH, but I don;t believe it is that basic.
Here is a link to a homebrew site that reported water for nearby Verona, WI. Note that the water is super hard (387 mg/L CaCO3) and has a high alkalinity (293 mg/L CaCO3), but pH 7.7:
https://www.homebrewtalk.com/threads/madison-wisconsin-water.211738/
Louis, in rye baking and in sourdough starters, TTA alone or TTA at certain pH level has meaning and importance, not pH alone. To each pH number corresponds a wide range of values of TTA and only when the target TTA is achieved, the starter is 'ready' (to be used, preserved, or to be fed) or the dough is 'ready' to be used in breadmaking - shaping, baking, etc. In those books that you quoted, it is assumed that your starter is the same as the author's starter and that in the time indicated it will reach the target TTA while fermenting at indicated temperature.
As Michael indicated in his comment below, pH in baking (or in beverage making) only reflects the astringency of the taste it helps other bakers know how harshly acidic the bread smells and tastes. pH reflects presence of strong acids in dough (or any other substance).
Acidity per se is Total acidity, the sum total of all acids in dough, strong and weak, otherwise known as total titratable acidity (TTA). It is as quick and easy to determine as pH and is cheaper than purchasing and calibrating pH meters. Once you determine it for your starter or certain bread dough you will know by the sum total of other characteristics how ripe sourdough or ripe bread dough looks, smells and tastes like at certain level of TTA. For example, once your sd starter reaches certain pH level, it might still take several hours of additional fermentation for it to reach the target TTA level and be ready to be fed again, or used in breadmaking.
Certain TTA is not just to slow down amylases which need time to work. Amylases do not destroy starches instantaneously, even in optimal conditions at very elevated temperatures that would kill all sd microorganisms it takes hours for amylases to digest starches. At room temperature amylases work very slowly and it takes many hours to accumulate acidity due to sd fermentation as ell.
Use of sourdough in rye baking has to do with the quality of the flour as is. When you moisten flour and immediately bake it, with or without yeast inside, you will see its falling number with your own eyes, because the ball of baked dough will look like in the pictures above when sliced open - gummy wet patches in the cut, huge cracks and empty spaces under the crust, etc.
In those quickly baked samples amylases have no time to work at all. It's the quality of flour itself (of the grain that was milled into flour) that requires the use of sourdough and a certain TTA value (sd starter and sd bread dough maturity) to make a good loaf of rye bread.
I bought that wine testing kit a while ago but I haven't got around to using it yet. I haven't been too concerned about TTA as my SD ryes have turned out pretty good lately.
I agree that it is cheaper than the pH meters. I also don't want the hassle of maintaining a pH meter after doing that for years in the chem lab.
Same here. I got mine years ago and used it very little, mostly for problem solving and quality control, especially with other people's sd starters and preferments. If you like your bread as is, then you do not need it at all.
Thanks for the link. Just purchased and will do some experimenting soon.
God for you, Troy! In addition to that you'll need a scale ( + - 0.1g) and distilled water (available in any grocery store). The measurement itself literally takes a minute. Very easy.
Today I baked my 60:40 rye:spelt loaf (will post in my blog in the next day or so). For the bake, I did the 2-stage rye sour build using only blueberry yeast water for leavening.
Stage 1 - 100g whole rye + 120g blueberry yeast water. Ferment 10 hours at 81 deg F
Stage 2 - 150g whole rye + 112.5g spring water + Stage 1. Ferment 4.5 hours at 81 deg F
At the end of Stage 2, the sour was roughly double in size with significant alveoli (should have taken a picture). I used dmsnyder’s “islands” indicator that the sour was ripe and ready for final mix. Aroma was a light fruity/apple with no noticeable “raw flour”. There also wasn’t much (if any) sour aroma.
For the test, 5g of rye sour was thoroughly mixed with 50g distilled water until all clumps were broken and any TTA was well dispersed. I let the mixture settle for 15 minutes. I then spooned out 10g of the supernatant (easier to see the end-point in the flour-free liquid and to use less sodium hydroxide) and titrated with the 0.2N sodium hydroxide. Titration end point was somewhere between 0.6-0.7g.
TTA = 0.6 * 5 * 4 = 12. So, for this bake using only yeast water, the final TTA for the rye sour build was in the 12-14 range.
For my next bake, I’ll use my whole rye starter and target an inoculation that will give me a ripe sour in about 10 hours at 81 deg F and 120% hydration. Guessing that will be something like 1:8.4:7 or 1:9.6:8. Anyone that regularly maintains a whole rye starter have any feedback on what this inoculation should be?
German SD recipes I have read typically use a 10% rye sour inoculation. It seems to be a good general amount and I have used that amount when improvising a bread recipe. My rye sour is maintained at 1:10:10.
My wife claims that I say things are simple, that aren’t really simple, so I had to laugh when Mariana dismissed my complaint about “calculating” TTA. While the test kit provides the chemicals and pipet needed to measure the drops into your liquified dough sample until the color change happens, it doesn’t (as noted) give you all the tools needed or very good instructions, especially when using dough.
My experience with TTA is from making home made vinegar and fermenting veggies for long term storage, where TTA is a matter of health and safety. Not so much an issue when making bread (unless you are a professional baker) where you will just wind up with a failed loaf.
I read a few articles on this, trying to understand the distinction.
https://bakerpedia.com/processes/titratable-acidity-tta/
https://metrohm.blog/2021/08/02/sourdough-ph-tta/
As near as I can tell:
Points (2) and (3) explain why (4) is relevant to breadmaking.
I was surprised by this because I had never seen any reference to TTA in baking books or videos. "The Rye Baker" (Ginsburg) and "The Bread Baker's Apprentice" (Reinhart) are for home bakers; I can understand that they wouldn't go to this level of complexity. But "Bread" (Hamelman) and "Baking and Pastry" (CIA textbook) don't mention it either. The Bakerpedia articles on TTA seem aimed at big industrial bakeries.
"Bread" and "The Rye Baker" do discuss how temp and time during fermentation can favor or inhibit yeast, lactic acid, or acetic acid; the Detmolder 3-stage process has one stage to promote each one. I suppose TTA, or the combination of pH and TTA could tell you about the amounts and ratios of the acids.
I wonder what the difference in taste is between disassociated (pH) acids and non-disassociated acids (the difference between pH and TTA). Maybe the non-disassociated acid flavors are more subtle, compared with straight-up H+ tart/sour.
It's an American thing. US authors tend to dumb things down. I think Advanced Bread and Pastry is the only book to date that dared to not to include cups and teaspoons. In authors' defence, this is somethingg that's most likely forced onto them by the publishers, but still. It also does not help any that rye is considered a niche, secondary thing and therefore poorly understood. And this is the part where I stopped, walked to my bookshelf, and grabbed the 3rd edition of Hamelman to check if he still says that white rye is generally unsuitable for baking. Does he? Oh yes, he does, even though white rye allows for some of the most spectacular breads out there. The bottom line is you won't find TTA in the US books, and in German and Russian books there's nothing else. But that, of course, requires access and the ability to wade through the language.
I'm disappointed that Hamelman couldn't just use all metric measurements (i.e, for the home baker) in his recipes when he issued the 3rd edition.
Americans have been extremely stubborn about adopting the metric system, but a baker that's shelling out big bucks for Hamelman's Bread is probably serious enough to use grams when measuring the materials.
As I said, I am 99% sure that he has no choice in this matter.
I've complained about traditional units in the home-scale recipes in "Bread". In practice I use the metric units and divide by 10 or 20. In the 3rd edition, the Workday 100% Whole Wheat recipe has only home scale measurements, metric and traditional. The measurements for the other recipes are the done the same way as in the 2nd edition; commercial quantity metric and tradition, and home size traditional only, sigh.
"The Rye Baker" shows metric and traditional units for home-size baking.
I have to agree with "Bread" and "The Rye Baker" that light rye is pretty much tasteless. Many Americans have bland tastes; light rye lets them order pastrami on rye without actually having to taste much rye. There is a similar situation with scotch whiskey. Apparently many Americans like the macho sound of "Gimme a scotch" but don't actually like the taste of scotch whiskey. Canny Scots distillers started making and selling "light scotches" several of which became best sellers in America.
I am partial to whole wheat and whole rye, but medium rye (like KA Organic Rye) isn't bad.
You can not judge it by deli rye, which is isn't even a rye bread.
What do you consider a rye bread, if deli rye doesn't make the cut (pun intended)?
"The Rye Baker" Old School Deli Rye calls for 40% white rye (I guess Ginsburg is more charitable to white rye than I remember; I used medium rye with a nice result).
"Bread" 3rd edition uses 15-20% medium rye Hamelman writes, "White rye flour is commonly used in this style of bread; it is, however, almost devoid of flavor." That's my experience at Katz's; best pastrami in the world but lousy cheap industrial rye bread. The pastrami at Langer's in Los Angeles is nearly as good as Katz's but the bread is much better.
It is surprising that TTA is not discussed more in the professional-aimed book like Bread. But I understand why it may be avoided in the more general home-baker oriented books.
I liked the Metrohm article as it had a nice concise discussion of pH and TTA. The author does seem to imply that the ratio of lactic/acetic acids can be determined by the TTA titration. There might be a subtle change during the titration that could allow inference of this ratio, but the ratio is normally determined using other instrumentation (chromatography) that is definitely outside the realm of the home (or professional) baker.
I also thought the Metrohm author's Butterzopf looked pretty fantastic, even though I know nothing about that style of bread.
The flavor/taste of the acetic acid/acetate or lactic acid/lactate will probably be perceived the same by our taste buds. Sodium acetate is often used to impart a vinegar flavor to foods (especially potato chips). And the intensity of that flavor is probably governed by TTA, the "zinginess" (technical term) by the pH, as stated by @mwilson elsewhere in this thread.
To say that PH has zero importance in rye baking since few (if any) bakers here have the tools or knowledge to calculate TTA values, and PH is their only tool. Personally, I use taste, but a PH meter is on my Christmas list.
I bake with sprouted and home ground Rye, not everyone in America uses store-bought Rye (or even has it available in their local grocery stores for that matter)
Great photo's BTW, the middle ones look like some of my first loaves ;0)
Actually, your taste buds are probably a good way to qualitatively measure TTA, because we can sense the relative amounts of the acids.
Vinegar has a pH of ≈2.4; a solution 20× more dilute is pH ≈ 3, ≈4× less acidic by pH. I'll bet you'll find the straight vinegar barely tolerable but the dilute solution pleasantly sour. I just tried it myself.
In dough, I suspect that your taste buds may be able to discern between levels of sour even if the pH hasn't changed much.
I think the current thinking is that taste detects something "in between" TTA and pH, i.e. both values affect the perception, and also the actual nature of the acids might influence it, perhaps because their smells are also different...
As part of my studies, in wine sensory analysis I had the pleasure to taste dilute glasses of acetic, tartaric, lactic, citric and succinic acid solutions.
TTA is key, it determines the intensity of sour.
pH is more the astringent quality of that sour.
And different acids have different qualities all in themselves.
Let me tell you tasting acetic acid was horrid, and as a volatile acid you could smell it coming! Surprise surprise as a wine imbiber, tartaric was the most pleasant. Citric and lactic were quite similar but lactic had a certain "roundness" (synesthetic) to it. Succinic was a bit weird, salty / ionic.
With SD breads, higher TTA means the more intensely sour it will be.
I'm surprised your comparison did not include malic acid.
I actually like the intense apple-y sour of malic acid. I add a small amount when I make cinnamon apple hot cereal with dried apples. It adds just a little zing to the flavor.
Despite my usually exemplary memory, I trick myself into thinking there were 5 acid samples.
In actual fact there were indeed 6 and malic was one of them! How did I forget that one!
Yes malic, apple like but of course it's the human element, the experienced quality is associative!
Imagine if you'd never eaten an apple in your life before...
Certainly the tartaric was wine-like!
Thanks, Ilya.
I was not aware that there was any current thinking with respect to TTA and pH. I would be interested in any references you might have. I'm not surprised that both are important.
I actually found an article specifically about SD bread, with the conclusion that pH is key to the sour perception! Haven't read in detail... But very quite unexpected! I wonder whether for some reason it's specific to bread.
https://pubmed.ncbi.nlm.nih.gov/32466901/
Too bad it's behind a paywall. I would love to read that one.
I have it, PM me for a copy.
history repeats itself: Can instrumental characterization help predicting sour taste perception of wheat sourdough bread? | The Fresh Loaf
I also have the full text, let me know if you still need it!
Thanks for the offer, Ilya, but I did get a copy.
I'm not sure I completely agree with the conclusions the authors made. Even though the strongest correlation to sour taste was pH, there were also strong correlations with TTA (r = 0.83) and lactic acid (r = 0.94). I think it's likely all are important for the taste of SD, like you had stated earlier.
While I love recipes, measurements and testing equipment, their primary purpose in my life are to allow me to experience the tastes, smells, feel and sight of the targets so I won't knead (snicker) them.
I learned the proper rye dough acidification through trial and error; It's to a point now where I change the amount I add to my dough based on how sour it tastes. I don't get a lot of ascetic acid in my sours, but when I do, it's a very different taste. I ferment a lot of different veggies, and the vinegary taste from them is VERY different from the sour taste I get from my preferments.
The problem is, you can't. That is you can when you are working with one sour, you have experience with it, and you know when it's sour enough, but it is fairly easy to be fooled by a sour with an unknown ratio of lactic and acetic acids. This is actually why and how faux sourdough exists.
Thank you for the detailed response!
How did you resolve the lack of TTA? Increased fermentation time regardless of pH, or doing something more like a 2-stage preferment, or ???
I'm a repeat offender of a Cruelty to Sourdough charge. I usually refresh my rye culture about once every two weeks, but have gone as long as six weeks without any ill effects. I do this not because I feel animosity toward it but just because that frequency fits my style of baking.
I refresh about once every two weeks with a 1:10:10 feeding. This corresponds to approximately the frequency of my rye bakes. I refresh 12–36 h before I need to use it a rye pre-ferment. I don't worry if it is past peak. I have also used it unfed in the King Arthur's Do-Nothing Sourdough and it worked just fine.
I really only wanted to maintain one sour culture. Because I wanted to use it mainly for high% rye breads, I decided a rye culture would be The One. That, and that rye cultures seemed to be easier to start and maintain. Both of those assumptions have been borne out in my rye culture. Starting my culture worked on the first try (Yay!). Between feedings, Vaal patiently lives in the fridge waiting for more food.
I will second @mariana's comments about pH and TTA. While pH is by far easier to measure than TTA, it is important that there is sufficient acidity for the dough. If your yeast water has that TTA, then it should be fine. I made Ginsberg's Pain au Cidre from The Rye Baker that used hard cider as the liquid for a dough containing 71% rye. It worked great and the flavor was excellent.
Wow! Thanks everyone for the responses…. I need to read through them all again but it sounds like the rye sour serves two purposes: minimizing starch attack (through pH) and developing flavor (through TTA).
At this point, I think I need to do a side-by-side test with my yeast water and my rye SD culture to see the difference. I think the yeast water has possibilities. The LAB’s are likely very low but the initial low pH of the water should help kickstart the LAB’s in the flour while suppressing the other nasties. I’ve never tried tasting my SD starter, but also sounds like something I should start doing.
This was an excellent thread, thanks to everyone who contributed to it.
Benny
Seconding Benny. This has been a really interesting discussion. Thanks to everyone for sharing.
I'm a thoroughly non-scientific rye baker & have never thought about the different roles played by pH & TAA. But I'd like to put a word in about my experience:
1. These days, I sample a dab of my rye sour every time I feed it. To my mind, the texture should be light and airy (an eater's version of the float test, I guess) and the taste should be sweetly sour -- a contradiction that, to my mind implies intriguingly tangy, but not so sharp that I can't taste the flavor of the grain.
2. I was interested to learn from Mariana's post that rye flour is both a good host for yeast and for bad bacterias. When I got back from a work trip in late October, I restored my rye sour with Bobs Red Mill dark rye. The bag was well within date and smelled OK, but the flour wouldn't hit the light/sweet notes I want in a starter. The bread it made was OK, but not great. So I got a few bags of a much fresher local brand called 'Farmer Ground Flour' and ... boom, the sour hit the sweet spot right away.
3. Now that the starter has recovered, I fed it 1:5:5, let it mull for a few hours, and stashed it in the fridge. My plan is to use what I need and refeed it when the stash has been mostly depleted -- probably after another 2 or 3 weeks.
4. As a side-note, I've been having other problems with bad flour lately. I baked a 90% whole wheat at my brother's house that was undone by an unopened 5-lb-bag of King Arthur White Whole Wheat that seemed to have gone to the dark side. It still had 4 months to go before the sell-by date. But the dough had a distinctly bitter undertone and so did the final loaf.
Thanks again for all the knowledge shared.
Rob
Sounds like both your rye and whole wheat went rancid. I vacuum pack my flour (among other things like nuts) to prevent that. If you're unlucky, sometimes you get it from the store like that ( justification for a return in my book.)
I've read a couple of times that while white flour can be stored at room temp, whole grain flour should ideally be stored in the freezer. Whole grain flours have a lot more fat/oil which can go rancid at room temp.
Unfortunately, my fridge is from 1949 and the freezer compartment is super-moist & doesn't fully freeze things. I should junk the antique, but I'm attached to it. From now on, when I want whole grains, I will exclusively buy local NYS 'Farmer Ground Flour,' which comes in 2 lb bags and seems fresher than mass distributed brands like BRM and KA. I'm not criticizing BRM or KA -- but just recognizing that they can't control how and for how long local supermarkets store their products.
Rob
Here are videos of people demonstrating the "simple" procedure for measuring TTA. I've never done it for sourdough and it was over 12 years ago that I did it with my daughter for vinegar and fermented vegetables.
https://www.youtube.com/watch?v=ZZmwt6Ug-Zg
https://www.youtube.com/watch?v=jPnhbr_z0EQ
https://www.youtube.com/watch?v=O6_PYhx9YyM
https://www.youtube.com/watch?v=hco8sufSWhE
https://www.youtube.com/watch?v=bzbqwaFHY5g
It's not that bad, nor scary.
The photo below shows how simple it is: starter, NaOH and indicator from the kit, 0.1g precision scale, water, dropper.
1) add a 5 g sample to 50g distilled water. It can be 5 g of starter, preferment, bread dough or bread crumb. The picture shows 5g of foaming liquid whole wheat sourdough starter floating on top of the water.
2) dissolve by stirring it a bit
3) add 2-3 drops of indicator (phenolphthalein). Tare to zero.
4) begin adding NaOH , little by little, mixing (use the dropper provided in the kit to draw NaOH from the bottle and to add NaOH to the solution on the scale), until light pink color appears. You are done!
The same process for a stiff rye starter.
See on the scale how many grams of 0.2N NaOH it took to neutralize all acids in that sample and multiply this number by 4 (x2 because it is only 50g not 100g g of water and another x2 because it is 0.2N, not 0.1N NaOH). This will be your sample's total titratable acidity value.
For example, the scale on the picture above shows that it took 4.4 g of NaOH to neutralize all acids in that sourdough starter's sample.
If it was neutralized by 0.1N NaOH, then TTA is 2x 4.4=8.8°. It is too low for any rye sd starter made from medium rye or whole rye.
If it was neutralized by 0.2N NaOH, as in the wine testing kit, then
TTA = 4x 4.4= 18°. This value would be normal for yeastless CLAS or FLAS, but abnormally high for yeasted starters.
Typical values for stiff whole wheat starters, 60-80% hydration
(1) young starter, pH= 4.5-4.7, TTA=6-9°
(2) mature or ripe starter, pH= 4.0-4.5, TTA=10-13°
(3) sour starter, pH<4.0, TTA>15°
Standard values of TTA for liquid and stiff starters
Stiff whole grain rye starters 13-16°
Stiff medium rye starters 12-14°
Liquid whole grain rye starters 10-13°
Liquid medium rye starters 9-12°
Stiff whole wheat starters 11-12°
Stiff white flour starters (all purpose, bread flour), 6-8°
Liquid whole wheat starters ≥7°, higher is better
Liquid high extraction wheat flour starters, 6-7.5°
Liquid white flour starters (all purpose white flour or bread flour, ash 0.4-0.7%), 4.5-5.5°
Hi Mariana,
Based on the inputs the TTA would indeed be 18 but your understanding here needs work. There are some underlying calculations that explain the theory of the assay which tend to get lost when it is described as a simple procedure.
E.g.:
C(a) x V(a) = C(b) x V(b)
C - Concentration
V - Volume
a - Acid
b - Base
Regarding your explanation the first thing to say is the actual quantity of distilled water added to the sample is actually not critical and therefore adjusting your calculation based on that is an error. The added water is there to help assist in the practicalities of performing the assay and doesn't affect the result.
The standard for TTA in the baking industry is to determine the degree of 0.1M NaOH needed to neutralise all acids in a 10 gram sample.
You were correct to adjust for the use of 0.2M NaOH but it is because of the 5g sample used in the example that explains the need to multiply by 2 once more.
For clarity and cohesion the TTA; this being the final calculation should be distinguished from the ‘titre’; the volume of NaOH recorded.
In the example above a titre of 4.4ml was determined where 0.2M NaOH was used to titrate a 5g sample. The TTA is therefore 18, as you correctly which is calculated with the formula below.
Standard TTA formula which assumes 0.1M NaOH is used.
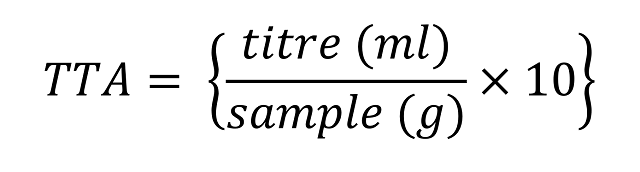
The following I adjusted to account for different concentrations of NaOH used.
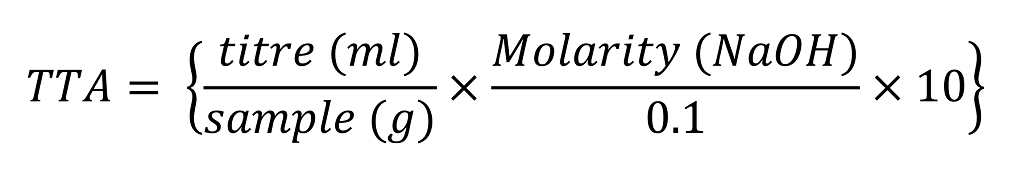
Hope this helps,
Michael
PS. I'm curious, what is the source of the TTA data you have provided?
Here is the link: https://www.mdpi.com/2304-8158/8/8/331/htm
Conclusions
Confirmed here: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6722811/
Which flours available to consumers best meet the criteria? I've been using KA Organic Rye as medium rye and Great River Milling Dark Rye in my culture and dark/whole rye recipes.
Rye flours aren't in that many stores; The Great River is in Sprouts (a small chain with a lot of organics); the medium rye I can find online only.
I've spent a good part of the last few days trying to find the answer to that question. I did come across a few mentions of a few Russian grains, and studies of how storage times affected flour quality, but nothing that directly answered that question.