Acid | |||||||
| H+ ions | Name | Base Formula | pKa1 | pKa2 | pKa3 | pH of 1mM | Molar mass (g/mol) |
| mono- | Lactic | C3H6O3 | 3.86 | 3.51 | 90 | ||
| mono- | Acetic | C2H4O2 | 4.76 | 3.91 | 60 | ||
| di- | Tartaric | C4H6O6 | 2.99 | 4.40 | 3.18 | 150 | |
| di- | Malic | C4H6O5 | 3.40 | 5.20 | 3.33 | 134 | |
| di- | Succinic | C4H6O4 | 4.21 | 5.64 | 3.65 | 118 | |
| tri- | Citric | C6H8O7 | 3.13 | 4.76 | 6.34 | 3.24 | 192 |
Lactic and acetic acids - by products of heterolactic fermentation e.g., sourdough cultures, pickles, cheese.
Lactic acid - Dairy fermentations e.g., cheese, sour milk, yoghurt, silage.
Acetic acid - vinegar, pickles, kombucha.
Malic, citric and tartaric - common fruit acids. Malic acid and citric are the most common major fruit acids. Tartaric is the principal acid of grapes and raisins. Citric is the major acid in citrus fruits.
Succinic acid - byproduct of yeast fermentation.
What effects do lactic and acetic acids have on dough? - both acids show a tightening effect on dough, or put another way, both acids can be shown to decrease extensibility of doughs. However, while lactic acid increases elasticity, acetic acid tends to increase resistance and make the gluten short and hard. The effect of the latter can cause gluten to rupture during dough handling or even as it rises.
Is there a point where pH begins to effect gluten structure regardless of enzyme activity? Do any of these get us there? I’m specifically looking at tartaric and it being dominant in raisins. A few times I’ve had doughs get really slack when I use a lot of RYW. I’m wondering if a different fruit source might help alleviate that. Maybe green apples or blueberries?
Hi Troy.
Yes, and yes would be the short answer with regard to the effect on gluten structure. All proteins are affected by pH and more broadly ionic interactions this being because proteins have dual charged sites (carboxylic and amino groups at their terminals) and so, as the environment changes, so do they (Tip: search for isoelectric point to learn more).
Gluten is however quite a complex in its makeup, structure and interaction with other flour components.
Gluten as you probably know is formed via two main proteins, glutenin and gliadin. Each of these proteins are defined differently according to their type. Glutenin is polymeric and is bracketed into HMW (high molecular weight) and LMW (low molecular weight) molecules. While gliadin is monomeric and has been defined into alpha, beta, gamma and omega molecular structures. And there are further layers of classes, such as whether they are sulfhydryl (thiol) containing...
And furthermore the structure of gluten is complexed by covalent, ionic and hydrogen chemical bonding interactions.
Gluten in an environment with pH lower than its native incurs swelling and this is maximised at pH 4.2 thus its takes on the most amount of water at this pH.
Let's look at the effect of pH on rheology.
With the farinograph, a key point of measure of interest is the stability. This figure roughly tells you how strong the flour is but also its tolerance to mixing and therefore fermentation capacity.
As the pH lowers so does the stability value (measured in minutes).
A lower pH than the native essentially means poorer performance. But that is one viewpoint. It is clear that there are further interactions of acidity such as coagulation and redox potential, and the types of acid involved.
I'm not a YW baker, so the practicalities I can't advise on but certainly I can relate having dabbled with it in the past and yes I too noticed considerable slackening (probably enzymatic proteolysis) and much ethyl acetate odour (often a result of stressed yeast), don't know if you experienced that too...
Of those acids listed tartaric is the strongest, meaning smaller doses have a greater influence on lowering the pH.
Regards,
Michael
(Apologies if my response is a bit scrappy, I am suffering vision problems and finding it hard to focus, so I might not be as succinct as I usually like to be)
Any chance you might want to add ascorbic acid to your list?
but this list was more about the kind of acids that one would encounter in significant quantities by way of sourdough and YW fermentations and then the ponderance of how they might affect gluten directly.
Ascorbic acid would only occur in infinitesimal amounts naturally which then may be oxidised rapidly unless kept in a closed system.
of Ascorbic acid has been shown to have significant effects on gluten development as evidenced by its addition to commercial flour at about .03% .
I said directly.
Redox is indirect
Thanks.
Aren’t there enzymes in flour that interfere with the oxidation of ascorbic acid and turn it into an oxidizer, explaining why it works as an additive for commercial millers?
Yes, but those flour enzymes actually invoke oxidation of ascorbic (AA) acid resulting in dehydroascorbic acid (DHA). There is some existing debate as to whether AA is oxidised directly by molecular oxygen or whether it occurs enzymatically.
Either way AA is oxidised rapidly, thus it can then act as an oxidiser in DHA form.
It's worth noting that AA once in liquid aqueous phase is very sensitive to oxidation with heat and light being the triggers.
A few years back a lot of people in the UK were reporting problems with their bread baking. Turns out because of bad weather the crop suffered. The advice everyone was given was to add lemon juice to the recipe and the problem was solved.
Not quite sure how this ties into the conversation but all this talk of citric acid reminded me.
That was to slow down the amylase reaction in “semi-sprouted” flour.
Using an online calculator I created data for solutions of lactic and acetic acid and graphed it.
What a weird smoother routine. Those steps can't be real. Also, can we have a link to the data source?
Granted, it looks funky. The smoother is excels default, I can turn it off but it won't look much different. The steps are the result of limited resolution on the pH data to one decimal place.
As I said the data I compiled by using an online calculator here: pH Calculator - Calculates pH of a Solution (webqc.org)
Data:
I can imagine a clean curved line through each but I couldn't figure out how to do that, perhaps you know how to?
Thanks for the data.
I can imagine a clean curved line through each but I couldn't figure out how to do that, perhaps you know how to?
I do - I'll work up a graph (not with Excel).
Here we are -
Image
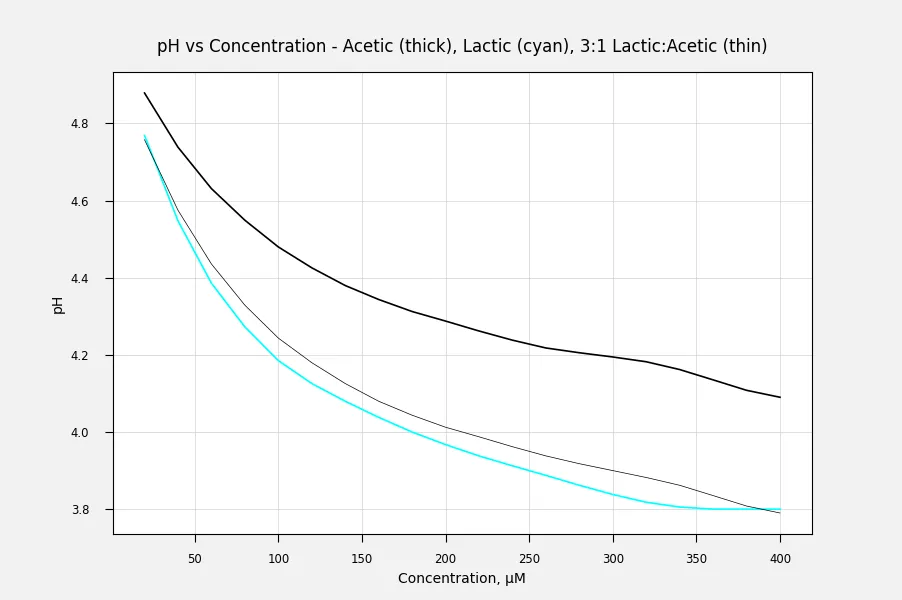
Thanks for that.
What software are you using to produce that graph? I'm interested to understand the mathematics involved. I presume the data points are being pulled up and pulled down to fit the curve.
The points are smoothed by an algorithm called LOWESS, for Locally Weighted Scatterplot Smoother. It would be more effective if there were more points, but even with so few it did a pretty good job.
LOWESS fits the points centered in a window with a weighted least squares routine, such that points farther from the window center are weighted lower. The window slides from point to point and the fit is computed for each window location. As long as there is enough data, and the data is halfway well behaved, LOWESS can do a surprisingly good job.
Here's an explanatory link-
https://www.itl.nist.gov/div898/handbook/pmd/section1/pmd144.htm
The software is my own GF4, which is a program to explore 2D data using an interface modeled after an HP Reverse Polish Notation (RPN) calculator except it works with curves instead of single numbers. I've made it available as an open source package -
https://github.com/tbpassin/gf4-project
Once I had your data table. I was able to paste it into the program and tell it which columns to use for each curve.
Here are the pH data calculated and plotted in Excel using the equation for pH from pKa and concentration. The mathematical solution for the single component acid is in most chemistry texts, but the combination of acids is more complicated. It's not just the sum of the pH of each acid.
The complication arises because the acids have relatively similar pKa (≈10× difference). Most examples of weak acid mixtures have one acid that is much weaker than the other, so it can be ignored in the calculation. With lactic and acetic, though, the lactic acid will ionize to a greater degree but this causes the equilibrium of the acetic acid dissociation to shift only slightly back to the acid form, instead of almost complete suppression.
I tried solving it myself, but my math abilities are much rustier than my chemistry. I finally found a solution online for this type of similar pKa mixture. The values for the mixture differ slightly from the above values. but without comparing the equations, I can't determine where the discrepancy is.
mwilson 's data make a nearly but not quite straight line when plotted with a logarithmic X axis. Since pH is a log scale, I figured that makes sense. The curves deviate near the start on the left, and also there are slightly odd deviations, especially for the Acetic Acid curve around a concentration of 300. I figured these were probably experimental issues. Maybe the pH sensor didn't behave quite correctly or maybe the chemicals weren't quite as clean as they could have been. Real-world issues, anyway.
If you would post the table of values in text form instead of an image I could overlay the calculations and measurements. It's not very important, but would be interesting (for two or three of us, anyway :-) ).
While I work on entering the values as text, here is some additional info on the calculations. I believe the pH data @mwilson posted is from an online calculator, not from pH measurements. I don't know what equation that calculator uses, but here are the equations you need to calculate the pH from concentration:
https://phcalculator.blogspot.com/2013/04/calculating-ph-of-monoprotic-weak-acid.html
Here is the equation for the mixture:
https://chemistry.stackexchange.com/questions/10249/how-to-determine-the-ph-of-a-mixture-of-two-weak-acids
Probably the reason you cannot fit the curve is that you're missing data; you need the concentration of the proton, [H+], in solution.
I forgot that Michael's numbers came from an online calculator, not actual data.
This is excellent, thanks for taking the time to put it together.
It's interesting to visualise how as the acids accumulate the pH rate of descension declines.
If the pH is proportional to the log of the concentration of Hydrogen ions (protons), and the concentration of Hydrogen ions is proportional to the concentration of the acid - which seems reasonable - then the curve of pH should be a straight line when plotted against the log of the concentration. When plotted against acid concentration on a linear scale, this will give the appearance of the slope leveling off.
These curves almost follow this pattern over most of the range.
I have a low opinion of high r^2 values since almost two things that go up or down together will have a fairly high r^2. It doesn't signify much. Since we're dealing with evaluations of a formula instead of experimental data, there's not much random measurement error, hence a high r^2.
TomP
Here is the plot of pH vs log[Acid]:
The linear trendlines added with Excel show R2 > 0.995 for all three.
Yup, pretty much the same as my program showed. And probably some of the small non-ideal behavior is nothing more than small numerical floating point issues.
I don't take much stock in large r^2 values since any two sets of points that go up or down more or less together will have a large r^2 value. And since these values represent the evaluation of a formula, there's no random experimental error to introduce any scatter. A high r^2 is practically guaranteed.
As an example of this r^2 behavior, construct a straight line of 100 points ranging between 0 and 1. Square it. The Pearson correlation coefficient between the straight line and the quadratic is 0.968. Yet the two curves deviate from each other quite a lot.
r^2 is more about scatter than smooth behavior.
As the concentration increases I would expect the relative influence on pH to weaken particularly from where the pH values are below the pKa of the acid. That appears to be the case from what I can see.
The effect is even more pronounced at much higher (millimolar) concentrations. Compare the dilute and not-so-dilute plots that now include the strong hydrochloric acid:
The strength of HCl vs the weak organic acids is really apparent. It's also interesting that in the µM range, the HCl solution isn't that much more acidic, not like the pH difference at higher concentrations.
I apologize to all other TFL members for the chemistry nerdiness of these posts!
Now go ahead and plot pH against the log concentration and even for HCl the line will most likely be nearly straight, just with a different slope from the others. I'd do it myself if I had the numbers, but I'm not inclined to scale them off the graphic. (I've done enough of that in my time).
The plot you suggest should be a straight line, especially for HCl.
By definition for strong acids, [H+] = [HCl], so pH = –log([HCl]. No complex math needed to determine the pH of a strong acid. You can just use any series of values from 0.001 M to 1 M to obtain the HCl plot.
Acetic acid Ka = 1.7539E-05; Lactic acid Ka = 1.38E-04
Data tables:
Acetic Acid Lactic Acid Mixture